Title: Probing Solvation-Induced Structural Changes in Conformationally Flexible Peptides: IR Spectroscopy of Gly3H+·(H2O)
Authors: Kaitlyn C. Fischer, Jonathan M. Voss, Jia Zhou, and Etienne Garand
Year: 2018
Journal: J. Phys. Chem. A
Doi: 10.1021/acs.jpca.8b07546
Amino acids are the building block of proteins (usually there are more than 50 per protein), a type of macromolecule that plays important roles in many vital aspects of an organism life, such as DNA replication, metabolism, signaling, transport. For the protein to fulfill its roles, the 3D shape (protein folding) that it assumes is critical.
How the protein spontaneously acquires its 3D structure from the amino acid sequence is still unknown, but it is understood that its structure depends on non-covalent bonds such as hydrogen-bonding, Van der Waals forces, ionic interactions, and hydrophobic packing.
The folding process is also affected by external factors, such as solvent, pH, and temperature. Hydrogen bonding with solvent molecules is deeply related to the functioning of the protein. When the protein folds in vivo (in an aqueous environment), it exposes its constituting hydrophilic amino acids (to the water) and “protects” its hydrophobic ones.
A deeper understanding of this interaction can help us understand how the proteins fold.
Conventionally, solution-phase spectroscopic is used to study proteins. However, it is difficult due to the complicated dimensions and the number of amino acids. Using gas-phase spectroscopy to study small peptides (short chains of amino acids) can help us to understand the simpler processes that occur, to get a better insight into more complicated ones.
In this paper, advanced gas-phase spectroscopic techniques (infrared predissociation (IRPD) and isomer-specific IR–IR double resonance and H2O/D2O substitution) are used to study thow one water molecule (H2O) induces structural changes over a small protonated peptide (triglycine, Gly3H+, Figure 1).
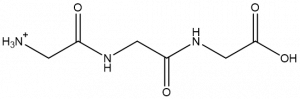
Figure 1: Gly3H+ 2D structure
An earlier study had shown that triglycine exists in the gas-phase mainly in two structures (Figure 2) in a 35:65 ratio (left to right), with unusual cis bonds -driven by hydrogen-bonding- and different from its structure in solution phase (in aqueous environment all the amide bonds assume trans conformation). [1]
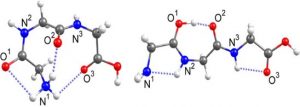
Figure 2: The two main gas-phase structures previously identified in a 35:65 ratio left to right.
Adapted with permission from Phys. Chem. A, 2018, 122 (41), pp 8213–8221. Copyright 2018 American Chemical Society
Performing these experiments in the gas phase allows researchers to isolate the ions from the bulk and gives the exact measure of how only one solvent molecule can influence the structure of these highly flexible peptides.
In a home-built instrument, the Gly3H+(H2O) ion aggregates are produced at 80° K isolated in the gas phase, cooled to a very low temperature (10° K) and their geometries are investigated via vibrational spectroscopy with the use of an IR laser (in the regions between 1000-2400 and 2400-3800 cm-1).
Despite this being a small system, complications in the spectra required the use of a second IR laser. Moreover, these results were compared to the spectra calculated with a computer software (Gaussian 16), to confirm the structures generating them.
When one water is added to the triglycine in the gas phase, the conformation of the peptide is modified, such that ⁓90 % of triglycin has trans bonds in solution phase (Figure 3).
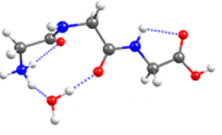
Figure 3: Most abundant Gly3H+(H2O) geometry.
Adapted with permission from Phys. Chem. A, 2018, 122 (41), pp 8213–8221. Copyright 2018 American Chemical Society
This paper shows how one water molecule is enough to cause structural changes of triglycine by hydrogen-bonding even at low temperature and how gas-phase vibrational spectroscopy is an optimal technique to understand the importance of these interactions.
Hopefully, this would further develop to study bigger peptides in the future that will help bring us a deeper understanding of protein folding.
[1] Voss, J. M.; Fischer, K. C.; Garand, E. Revealing the Structure of Isolated Peptides: IR-IR Predissociation Spectroscopy of Protonated Triglycine Isomers. J. Mol. Spectrosc. 2018, 347, 28−34.