Featured image used with permission from Pixabay
Paper: Predicting the Time of Entry of Nanoparticles in Lipid Membranes
Authors: Changjiang Liu, Paolo Elvati, Sagardip Majumder, Yichun Wang, Allen P. Liu, and Angela Violi
Doctors want to use tiny crystals called nanoparticles to deliver drugs straight to the part of your body that needs them – minimizing any harmful side effects and making medicine more effective. But how can nanocrystals travel through your body to the right location? Researchers at the University of Michigan have come up with a theoretical model that examines one part of this complex problem: what happens to nanoparticles when they cross lipid membranes.
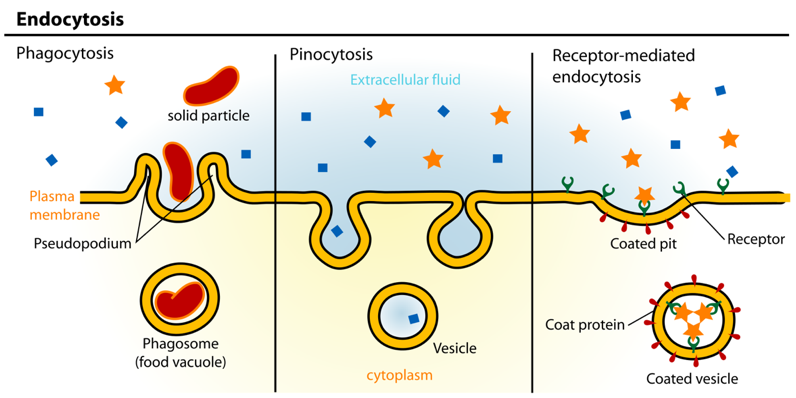
Lipid membranes, as you might remember from biology class, surround both the cells in your body and the structures, like the nucleus, within your cells. Lipid membranes control the movement of proteins and other molecules in your body. Smaller nanoparticles can cross lipid membranes by diffusion, a form of passive transport. Larger nanoparticles usually cross by physically penetrating the lipid membrane (membrane deformation) or by endocytosis, where the membrane surrounds the particle, then breaks this off as a vesicle inside the cell (Figure 1).
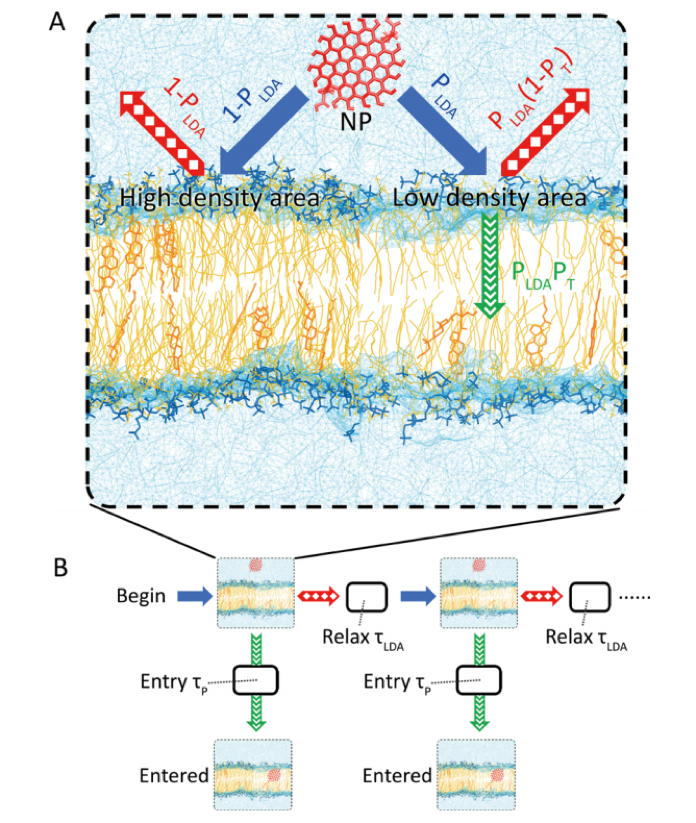
The different properties of the nanoparticle – including its composition, shape, and surface chemistry – can also affect how it diffuses across a lipid membrane. In order to quantify how these properties affect the diffusion of different kinds of nanoparticles across lipid membranes, the researchers created a model to measure the time it takes a nanocrystal to penetrate the hydrophobic center of the lipid membrane – the “time of entry”.
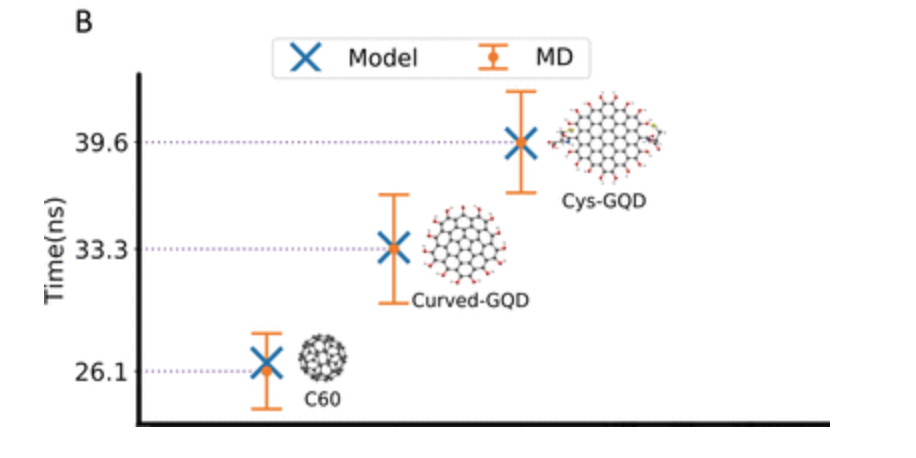
The researchers chose to study carbon nanoparticles, as these are commonly used in medicine. They studied nanoparticles of different shapes and hydrophilicities while keeping the sizes similar. Buckminsterfullerene and graphene quantum dots were chosen to vary the nanoparticle shape; graphene quantum dots with either –OH or cysteine groups on the surface changed the hydrophilicity of the nanoparticle surface.
In order to model the movement of the nanoparticle through a lipid membrane, the researchers divided the process into 4 steps. First, the nanoparticle must diffuse to the surface of the membrane; next, the nanoparticle diffuses over the membrane before entering the outer hydrophilic region of the membrane. Finally, the nanoparticle enters the hydrophobic region of the membrane. The researchers only measured the time for the nanoparticle to enter the lipid membrane.
Using simulations of the movement of the molecules involved (molecular dynamics), the researchers computed the probabilities of the steps occurring for the different sizes and shapes of nanoparticle. They found that the small buckminsterfullerene nanoparticles entered the lipid membrane more quickly than either of the larger graphene quantum dots, regardless of whether they were terminated with –OH or cysteine molecules; these results agreed with their model within 10%.
With this model, nanoparticles could be designed to deliver drugs to specific sites in the body based on the composition of the membrane, allowing much lower dosages of drugs to be used – helping eliminate unwanted side effects. New drugs could be screened quickly and efficiently without wasting laboratory resources. And, of course, there are always further refinements to be made to the model itself. While this model is designed to guide future research rather than suggesting new strategies for nanoparticle drug delivery, its success means it could be very useful in medical research.