Article Title: A Single-Flow Battery with Multiphase Flow
Authors: Lihi Amit, Danny Naar, Robert Gloukhovski, Gerardo Jose la O’, and Matthew E. Suss
Journal: ChemSusChem.
Year: 2021
Title: Zinc bromide battery works without membrane by using bromide complexing agents
Solar and wind energy are continually increasing in prevalence because of their decreasing costs and lack of carbon-emissions during operation. However, these energy sources are inherently intermittent and energy storage solutions must be developed for periods when the sun and wind are not available to meet energy demand. Redox flow batteries operate by storing dissolved electroactive materials, which are usually metals or small molecules that can store and transfer electrons, in two tanks and pumping it past an electrode to store and release electricity. Traditionally, vanadium metal has been used for the electroactive material and an ion-exchange membrane has been placed in the center of the battery to prevent the charged electroactive materials from crossing over to the other size.
Vanadium, however, is expensive, so researchers from Israel Institute of Technology designed a battery using bromide (Br2) and zinc, a much cheaper metal, as electroactive materials instead. Since zinc is a solid, it has a tendency to form dendrites, which are long skinny protrusions of solid zinc, as the battery is charged and discharged. This eventually degrades the battery at the dendrites reach across the battery and cause it to short-circuit. Because bromine causes rapid corrosion, interaction of bromine with the zinc will accelerate the process, so it is important to keep the zinc and bromide on separate sides of the battery.
Ion-selective membranes are expensive and compose a large portion of the cost of a typical redox flow battery. To address this, researchers developed a membrane-free flow battery where the two electrolyte solutions are kept separate by slow diffusion between them. To facilitate this, they used bromide complexing agents including quaternary ammonium bromide salts, which are a positively charged nitrogen atom attached to four carbon atoms (for an example, see Figure 2). This held bromine atoms in a polybromide phase with between three and 7 bromine atoms held by each nitrogen. This polybromide phase tended to float to the bottom of the tank, leaving a smaller concentration of active non-complexed Br2 above it. Since there is less free Br2 ¬available, it is slower to cross to the zinc side of the battery even though no membrane is used. The design of the battery is shown in Figure 1.
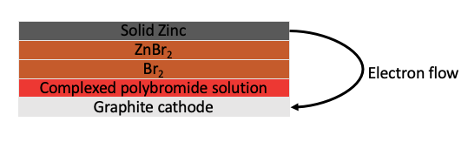
Figure 1: The complexed polybromide tends to separate from the aqueous Br2 layer and form an oily red layer on the bottom. When the cell is pumped, it tends to form globules around bits of aqueous bromide solution. This keeps the Br2 concentration low and minimizes the amount of bromide that reaches and corrodes the zinc electrode.
For the bromide complexing agent, the researchers chose to use 1-Ethyl-1-methylpyrrolidinium bromide, abbreviated to MEP (Figure 2). This compound was chosen because it forms a relatively weak association with bromine and easily releases Br2 at battery operating temperatures of around and slightly above room temperature. In the battery, bubbles of the oily MEP phase could be seen surrounding aqueous Br2 solution.
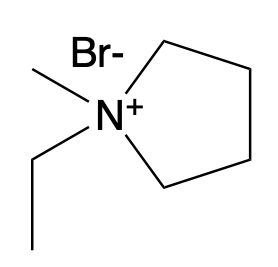
Figure 2: MEP, shown above, holds many polybromide solutions away from the aqueous Br2 solution.
When the volume of the polybromide MEP phase was low, the cell had a relatively high voltage of 1.74 V. However, the low Br2 concentration limited the current density to relatively low currents of 60 to 90 mA/cm2 by limiting the rate at which the free Br2 could reach the electrode to discharge. When the researchers increased the volume of the polybromide phase by a factor of five, this mass transfer limitation disappeared and current densities as high as 270 mA/cm2 were achieved.
The plating efficiency, or the mass of zinc at the end of a cell cycle compared to the mass you would expect if all electrons were successfully transferred when the battery was charged, measures how efficient the battery is at using electrons to store and release energy. In this battery, was 74% at a current density of 270 mA/cm2. The plating efficiency decreased when the volume of the polybromide phase was increased or when the charging was carried out over a longer time, which suggests that the Br2 in solution may be causing the decrease in efficiency by corroding the zinc. The zinc was smooth after charging and discharging, however, indicating that the dendrites were not forming rapidly, resulting in much slower battery decomposition.
The authors suggest that future work might explore more tightly complexing agents for bromide. This would have the effect of leaving less free Br2 in the aqueous phase of the battery and slowing corrosion caused by the bromide. If a membrane-less battery resistant to corrosion could be developed and scaled up, it could significantly decrease the capital cost of redox flow batteries.
