Title: Atmospheric- and Low-Level Methane Abatement via an Earth Abundant Catalyst
Authors: Rebecca J. Brenneis, Eric P. Johnson, Wenbo Shi, and Desiree L. Plata
Journal: ACS Environmental Au 0, 0, pp 2021.
DOI: 10.1021/acsenvironau.1c00034
Featured image by Danielle Gruen
—
Methane is an incredibly potent greenhouse gas that has gained more attention in the past few years. Recently, the United Nations has created a call to action to reduce methane emissions and slow global temperature rises. Methane is of concern because its potential to absorb heat in the atmosphere (radiative forcing) is 120 times greater than carbon dioxide immediately after being emitted. Even over 100 years its average effect is 30 times greater than carbon dioxide (the diminishing effect with time is due to the oxidation of methane to carbon dioxide in the atmosphere. Researchers at MIT have found an unconventional solution to mitigate methane’s impact by converting methane to carbon dioxide. While it seems unproductive to convert methane to carbon dioxide since carbon dioxide is the most notorious greenhouse gas, the warming that can be avoided by converting methane to carbon dioxide in the near term is tremendous. Converting methane to carbon dioxide is nothing new (burning natural gas is a key example) but methane at ambient concentrations is especially pernicious. Methane at concentrations less than 4% cannot be flared, which meant until recently we had no way to deal with most of the world’s methane emissions. A study by Rebecca Brenneis and colleagues have developed a novel method for methane to carbon dioxide conversion by using an earth-abundant catalyst that operates at relatively low temperatures.
Brenneis begins by discussing the preparation of the catalyst which was synthesized by taking a zeolite (microporous aluminosilicate) powder and doping it with copper. The experimental setup consisted of a tube furnace reactor (a well-controlled continuous plug flow reactor) packed with the catalyst. The gases were premixed to represent atmospheric concentrations of species and delivered to the reactor at a flow rate that produced a residence time (the average time each molecule spends in the reactor) of 30 seconds. The products of the reaction were then analyzed using a gas chromatograph.
The rest of the paper focuses on dialing in the appropriate activation and reaction procedure. The first scheme, A two-step activation-then-reaction, is described first because it is consistent with other methane conversion literature. Activation of the catalyst primes the catalyst and provides better conversion efficiency. Brenneis showed that this two-step process required high activation temperatures (500 C) to when paired with low reaction temperatures (200 C) to get good conversion efficiency (~90%). She also showed that the activation required up to 8 hours to obtain good conversion. This is hypothesized to be due to constrained gas transport or structural rearrangement of the copper. However, the conversion efficiency shows slight improvement over multiple activations.
The second scheme, Brenneis and colleagues developed was a methane conversion approach that used less energy by keeping the reaction and activation temperatures the same. This scheme is referred to as isothermal because the activation and reaction temperatures are the same. Figure 1a shows the matrix of reaction temperatures and activation temperatures against conversion efficiency, representing the two-step scheme. Figure 1b shows the isothermal activation and reaction against conversion efficiency. It can be seen from this plot that above 300 C the isothermal process results in remarkably high conversion efficiencies.
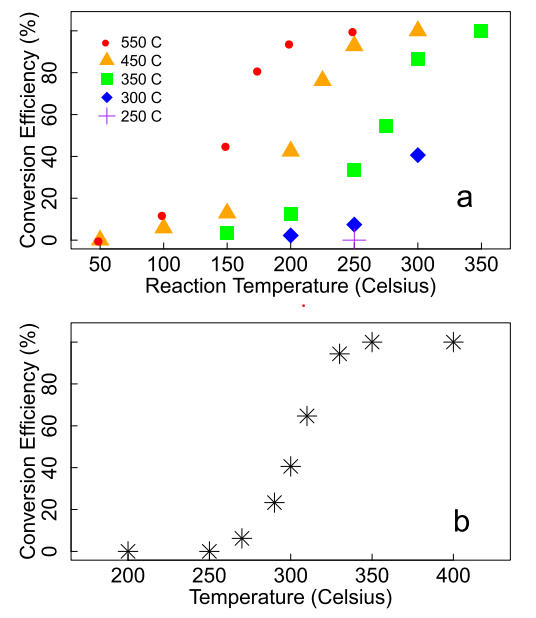
Figure 1: a. Two-step activation-then-reaction process. The activation temperatures are shown in the key and the reaction temperatures on the x-axis. b. Isothermal process. The activation temperature and reactions temperatures are the same and both shown on the x-axis.
The isothermal process also extends the lifetime of the catalyst when compared with the two-step process. The two-step activation followed by reaction resulted in a rapid deterioration of the conversion efficiency while the isothermal process resulted in steady high conversion efficiency for 12 days. The isothermal process is hypothesized to be continually renewing and reactivating.
The isothermal process is very advantageous from an energy efficiency perspective since there does not need to be two separate cycles that involve excessive power in heating and cooling. Brenneis also states that since this process is exothermic some of the heat could be recycled and used for energy generation, providing greater energy and environmental efficiency
Brenneis concludes that optimization can be done around activation and reactor geometry which could lead to higher conversion rates. However, further investigations are required as the catalyst still needs to be examined under high humidity and high volatile organic compound concentration conditions.
It is critical that methane emissions are brought under control quickly to mitigate climate change. Unfortunately, there are no off-the-shelf options currently deployable to handle diffuse methane. However, there is exciting potential for this technology to be implemented in the field either at dairy farms (to convert methane from cow burbs) or coal mines (where fugitive methane is released). This research is crucial in the path to ambient level methane abatement.

