Title: Dynamic Spin-Controlled Enantioselective Catalytic Chiral Reactions
Authors: T. S. Metzger, R. Siam, Y. Kolodny, N. Goren, N. Sukenik, S. Yochelis, R. Abu-Reziq, D. Avnir, and Y. Paltiel
Journal: The Journal of Physical Chemistry Letters
Year: 2021
Featured image from Metzger et al.
Prior to the 1920s, the understanding of the structure of matter and its properties used to be modelled by classical physics. Concepts including mass and charge ruled early theories trying to explain the behaviour of matter. However, when quantum mechanics was developed, we realized that subatomic particles, particularly electrons, play a fundamental role in the properties of materials.
A notable quantum property of electrons is their spin. Electrons have two allowed spin states, commonly known as spin up and spin down. Under normal conditions (i.e. no applied magnetic field) these states are degenerate (have the same energy). However, if a magnetic field is applied to a system containing electrons, the energy of their spin states will split.
To better understand this effect, you can think of an electron as a fish, swimming in a pond. It takes the same amount of energy for the fish to swim in any direction in the still water, just like an electron when there is no magnetic field being applied. Both the fish and the electron have degenerate energy states. Now consider a fish in a flowing stream. The rush of water around the fish is analogous to applying a magnetic field around an electron. In this case, it takes more energy for the fish to swim against the current than with the current. Thus, the energy levels will split into two non-degenerate states. For the electron, there are two spin states (up and down), one high energy and one low, depending on the direction of the magnetic field.
The effect that a magnetic field has on an electron can have major implications when it comes to electron transfer through chiral molecules (Figure 1). Such molecules are characterized by their mirror images not being superimposable with the original, just like your right hand is not superimposable with your left one. Each version of the molecule is called an enantiomer. Other important examples of chirality are helical molecules such as helicene and proteins, which can curl either clockwise or counter-clockwise.
When electrons move through a chiral system, they feel a well-oriented magnetic field acting on them. Importantly, this magnetic field acts on the spin of the electron causing preferential transfer of electrons with a particular spin (spin up vs. spin down), depending on the enantiomer of the chiral system. For instance, if we go back to our fish example, we can think of enantiomers as rivers having either an upstream or a downstream current (a well-oriented magnetic field pointing up or down) and electrons as fish trying to go either way in the stream (spin up or spin down). Clearly, downstream-travelling fish will have an easier time going through a downstream flowing river and vice versa. Following the same principle, electrons with the proper spin will travel preferentially through one enantiomer, which acts as a spin filter. This is referred to as the chiral-induced spin selectivity effect (CISS).
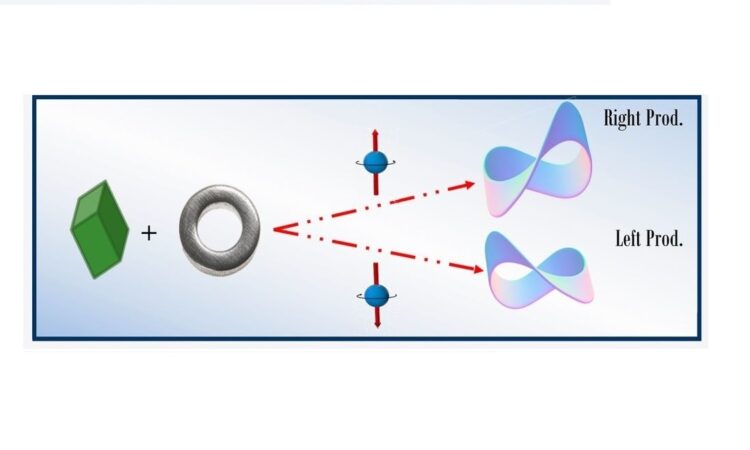
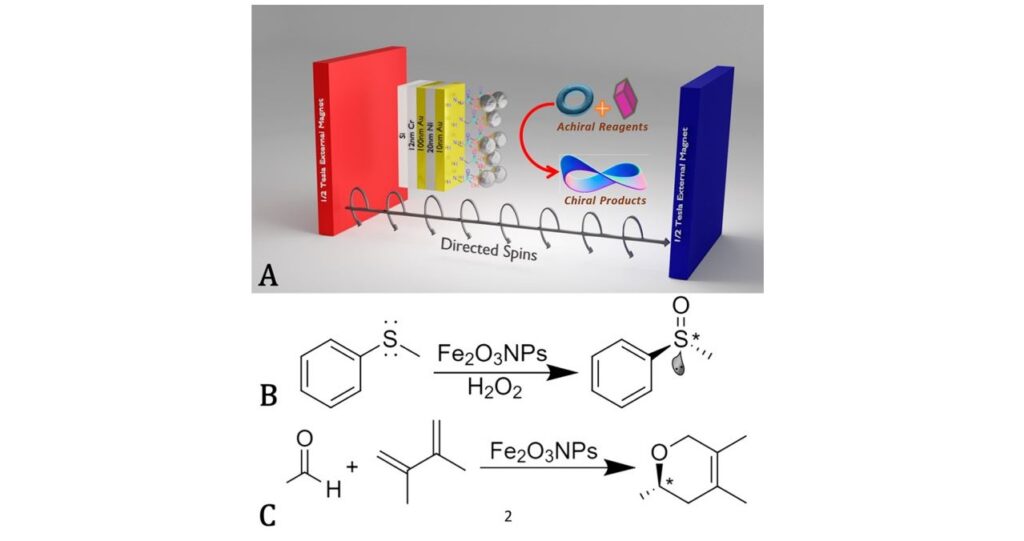
Metzger and co-workers have demonstrated the practicality of the CISS effect by performing chemical reactions that favour the synthesis of one enantiomer molecule through selective electron transfer in two chiral systems (asymmetric catalysis). The researchers created chiral products from precursors without any chirality (Figure 2) and the percentages of their chiral products were measured at reaction completion. The substrate was composed of nickel (ferromagnetic) to control the direction of the magnetization and to promote the production of one enantiomer at the surface. In addition, two metal plates surrounding the reaction vessel provided a constant magnetic field to control the orientation of the electron spin at the surface. The transformation from non-chiral to chiral happens on Fe2O3 particles attached to the substrate in the presence of hydrogen peroxide. Through optical methods, the group showed that they could stimulate the preferential formation of either enantiomer by switching the magnetic field direction. This demonstrates the significance of the CISS effect for real-world applications including the synthesis of important medicinal chiral compounds such as ibuprofen and ketamine.
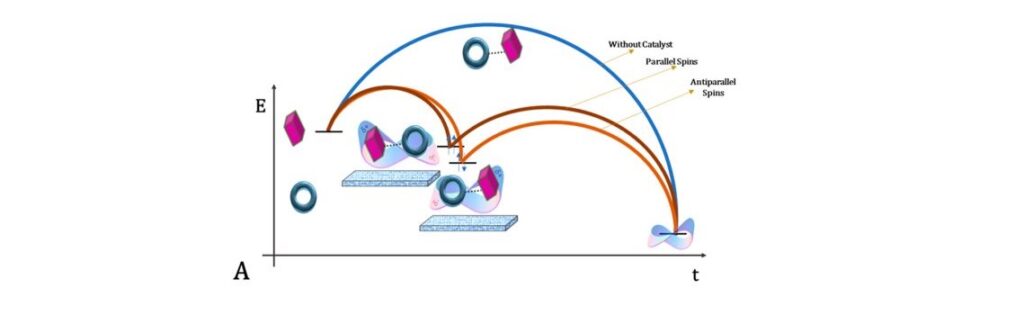
The proposed reaction mechanism is depicted in Figure 3 and consists of three steps. 1) The substrate provokes a redistribution of electrons in two precursor molecules (pink rectangle and blue ring, Figure 3); 2) A chiral intermediate state is formed, and its spin energy states split (parallel and antiparallel, Figure 3) due to the applied magnetic field and the nature of the surface; 3) The reaction proceeds through the lower energy pathway (antiparallel spin) because of the selective spin-based electron transfer as per CISS effect. Therefore, the production of one of the enantiomers is efficiently bolstered by harvesting the spin filtering capabilities allowed by the CISS effect.
The field of spin selective chemistry is still in its infancy. However, the research described here is one of the first of its kind and emphasizes how crucial it is to understand the behaviour of materials at a subatomic level to create meaningful macroscopic advances including asymmetric catalysis and spintronic technologies. This group was able to show a proof-of-concept use of the CISS effect in the asymmetric catalysis of two achiral systems into chiral products. This work paves the way for a much broader implementation of the CISS effect avoiding the use of external aids to achieve a spin selective electron transfer.