Authors: Aran Insausti, Jiarui Ma, Qian Yang, Dr. Fan Xie, Prof. Dr. Yunjie
Journal: ChemPhysChem
Year: 2022
Featured image modified from Swap Icon by Daniel Bruce on IconScout
When we see pictures of molecules, they are usually still. However, in real life, molecules are in constant motion: not only moving throughout their environment, but also internally. You can picture a molecule like an amusement park ride — it rocks back and forth, spins all the cars together, and each car spins individually. When we describe them, we don’t consider this movement, which means we are missing the full picture.
As molecules move, they crash into each other, and if the conditions are right, they react. Atoms can be pulled from one molecule to another, transforming it from a reactant into a product. The simplest of reactions is called a hydrogen abstraction — a single proton, a hydrogen nucleus, moves from one molecule to another. Investigating the conditions of hydrogen abstraction is an important question for chemists who want to know the very, very basics of reactions.
The authors of the study Insausti et. al. looked at the dimer, or weakly bonded pair, of 2-furoic acid. 2-furoic acid is a small carboxylic acid used as a food flavoring and preservative . A carboxylic acid is a molecule that has a carbon bound to two oxygens, one of which is attached to a hydrogen. The hydrogen is particularly reactive because the oxygens attract its electron strongly.
The molecule can take on different shapes, called conformers, which change the likelihood of it reacting (Figure 1A). Conformers in 2-furoic acid depend on the placement of the hydrogen relative to the rest of the molecule. When two molecules have carboxylic acid groups, the hydrogens can form connections called hydrogen bonds to the oxygen on the other molecule. If the right amount of energy is absorbed by the dimer, the hydrogens will undergo a double hydrogen abstraction and swap atoms (Figure 1B).
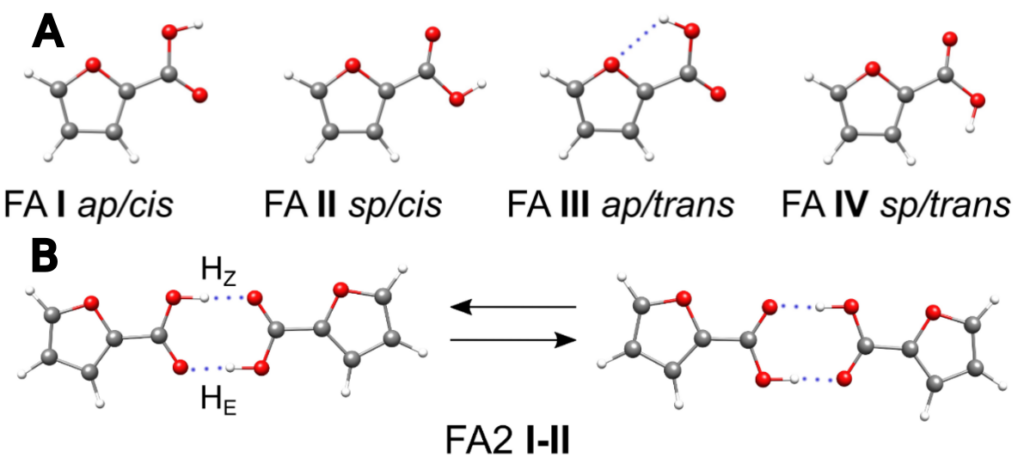
Because the molecules are held together by hydrogen bonds, they can be treated as a single molecule for a measurement technique called rotational spectroscopy. Rotational spectroscopy measures the energy differences between a molecule’s fixed rotation speeds. As every change in the shape of a molecule changes the rotational spectrum, and even tiny details of molecules can be explored. The technique works a little like a tuning fork near a piano. The piano plays multiple notes at once, and the tuning fork begins to vibrate at one of the frequencies the piano plays. Similarly, scientists shine microwave light of a variety of energies on the molecules and measure the frequencies they resonate with. The energies that correspond to a rotational change that molecule can undergo are extremely specific to that molecule in that specific molecular position. Scientists first calculate all of the possible transitions, then compare them to the real spectra taken to identify which signals correspond to which quantum changes. Unfortunately, two of the three most common 2-furoic acid dimers can’t be observed with rotational spectroscopy because they are too symmetric. Therefore, this analysis is only on the third dimer, which is unbalanced enough that we can see the rotational transitions.
The hydrogen swapping creates two very similar energy states that we can see on the spectrum as splitting — one big line is split into two lines that are very close, and that pattern is repeated (Figure 2). Each energy is split into halves depending on the exact state of the hydrogen swapping.
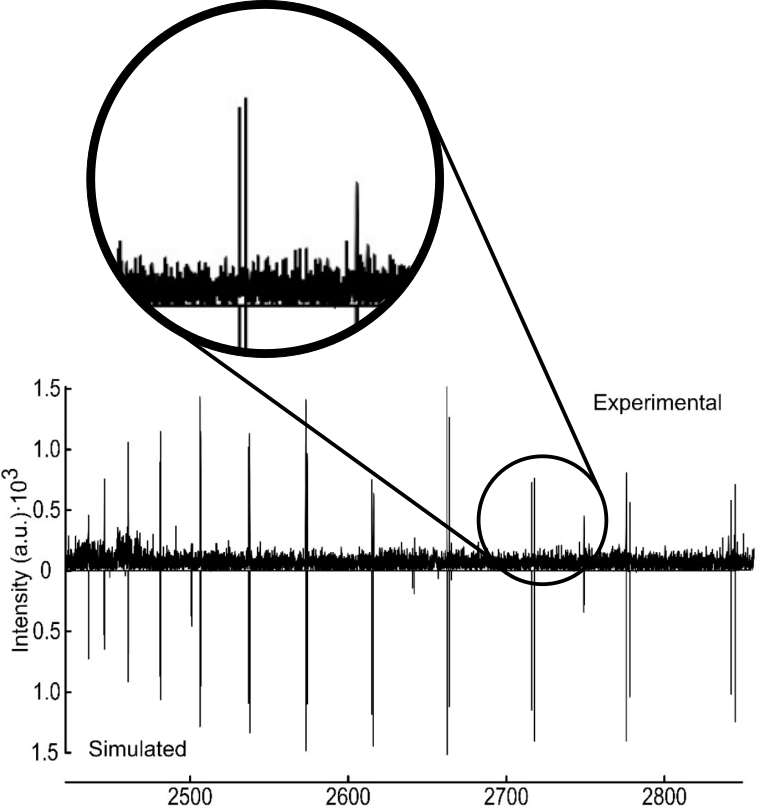
The energy difference between these states, called the ΔE01, is 1056 MHz — about half the energy of the radiation used by a microwave oven. The most interesting question is how difficult the transition is. How much energy does it take the molecule to be able to swap these two hydrogens? That value is called the energy barrier and is fundamental to all questions about reactivity. Just like a ball must have a certain amount of speed to get over a hill, molecules require energy than the energy barrier to react. The energy difference measured from the spectrum was put through complex quantum chemical math. The result was an energy barrier of 442 cm-1 — the dimer needs to have that much energy to swap hydrogens. This knowledge brings us ever closer to fully understanding how reactions happen at a very fundamental level.
| Article Title | Rotational Spectroscopy of 2-Furoic Acid and Its Dimer: Conformational Distribution and Double Proton Tunneling |
| Authors | Aran Insausti, Jiarui Ma, Qian Yang, Dr. Fan Xie, Prof. Dr. Yunjie Xu |
| Journal | ChemPhysChem |
| Year Published | 2022 |
| Link to Text | https://doi.org/10.1002/cphc.202200176 |
