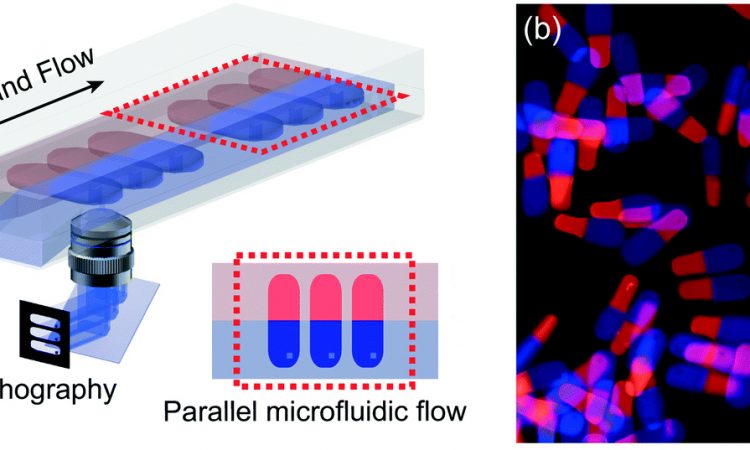
Tailoring treatment for a specific patient is the future of medicine. Let’s learn about making tiny pills that are “smart” enough to know where to dissolve in the body!
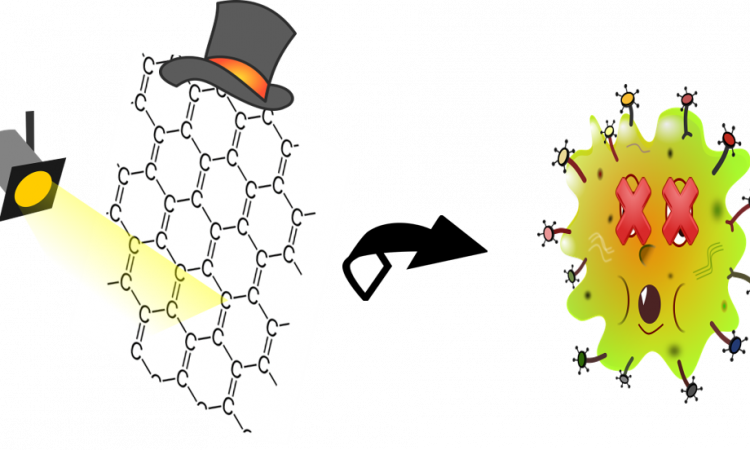
The Thousand Wonders of Graphene: from 2D to 3D Photodrugs!
The authors of this paper can make a photodrug from a special type of graphene.
How to Prevent Cannibalism in Catalysts
This work describes an approach to prevent self assimilation of catalysts to increase their lifetime. It also finds a Hammett correlation between different substituents present on the catalysts and the rate of catalysis in both homogeneous and heterogeneous phase.
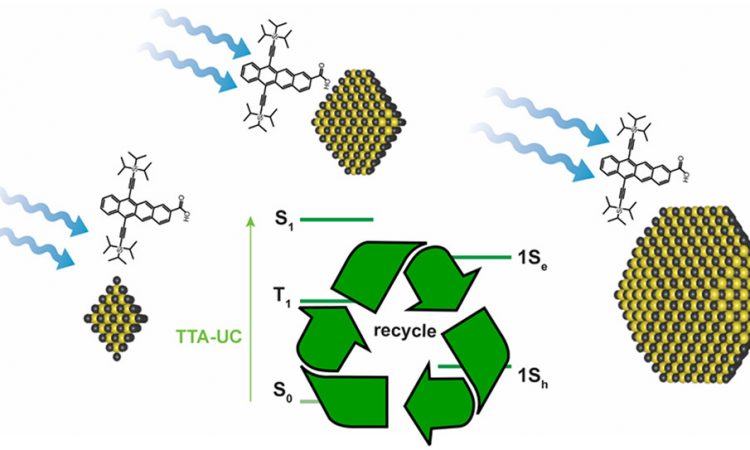
Energy Flow in Quantum Dot – Organic semiconductors
Quantum dots are fascinating super small solids. Highly conjugated tetracene is an electronically active organic molecule. When these two are mixed, electrons bounce around in amazing ways and these researchers found out how.
Flexible iPhones and Wearable Touch Screens: Transparent Conductors with Silver Nanowires
Flexible touch screens and see-through electronics could be closer than you think! Let’s learn about a new way to make transparent conductors with silver nanowires!
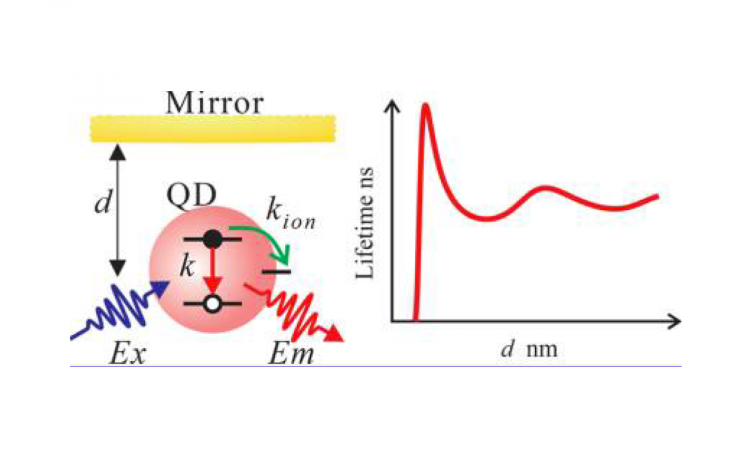
Nanoparticles and Mirrors: A “Bright” Future for Nanocrystal Blinking
You probably look in a mirror every morning: fix your hair, maybe even take a selfie. But the idea of using mirrors to look at molecules – that just sounds crazy, right? Maybe not – but you’ll have to read this Chembite to find out!
Always Use Protection: Preventing Catalyst Degradation with Coatings
Catalysts are critical components of many industrial processes. Unfortunately, many promising catalysts degrade over time. Here, researchers show that some catalysts can be protected by coating them with another material.
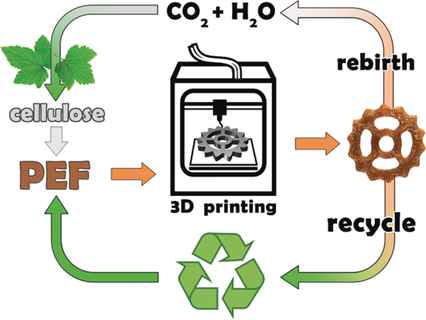
Green 3D printing: from cellulose to plastic objects!
3D-printing at its greenest!
A resistant material for 3D-printing is synthesized from plant components!
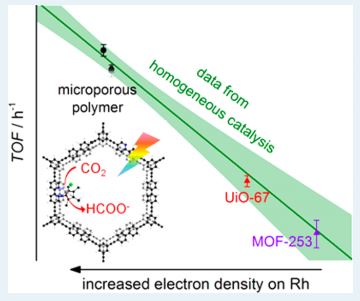
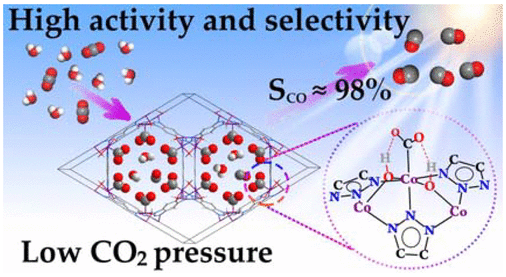
Structure-Function Relationship in a Highly Efficient CO2 Reducing MOF
A structure-function relationship has been established for a cobalt containing Metal Organic Framework (MOF) that catalyzes carbon dioxide reduction very efficiently. It has been established that the hydroxyl groups coordinated to the metal co-operates to enhance the catalysis by forming H-bond network with CO2. Let’s learn how the authors performed a systematic and thorough investigation on these MOFs.
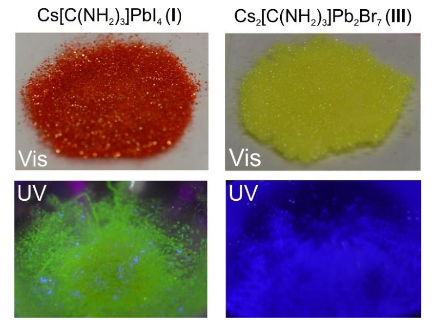
Designing and Understanding New Semiconductor Crystals
Solar panels are expensive because of the high-purity silicon present in them. A new material called perovskite rivals the solar conversion efficiency of silicon, but at a fraction of the cost. There is however still a lot of fundamental understanding to be done on perovskites, which these researchers do by studying analogous structures.
Looking inside your TV
Ever wondered how the images on your TV or computer screen are formed? Today let’s look inside your TV and learn about the nanoparticles forming the high definition display! (Obviously without slicing it open!)
Popcorn supercapacitors – future batteries for electric cars?
Most people prefer their popcorn popped to perfection, but scientist Jianhua Hou prefers his burnt. How could the smell of burnt popcorn possibly be a good thing? Chembites investigates!
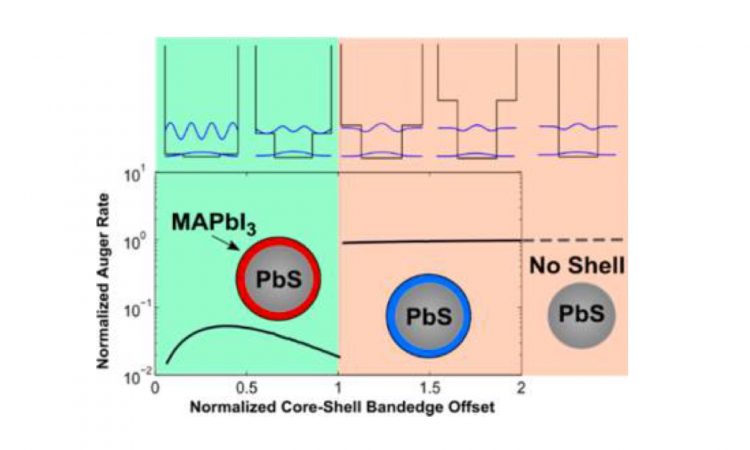
Quantum Dot Lasers: Improving Efficiency with Perovskite Shells
Lasers are cool – everyone who’s seen a sci-fi movie knows that. But we still haven’t figured out how to use them to their full potential in real life. This paper explores some ways to improve the efficiency of quantum dot lasers, which have a myriad of applications in computers to cell imaging.
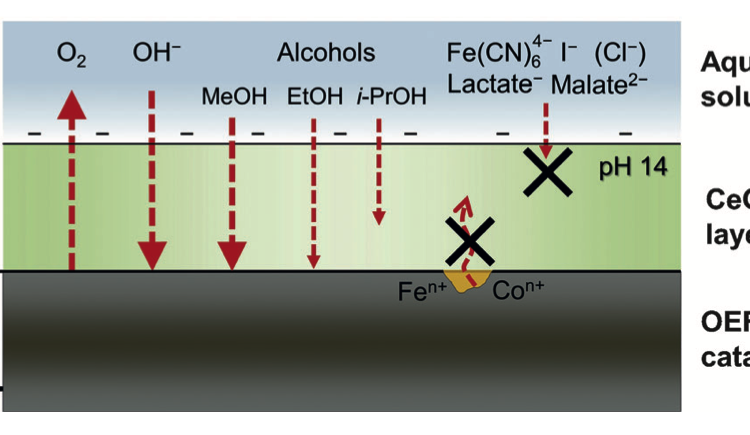
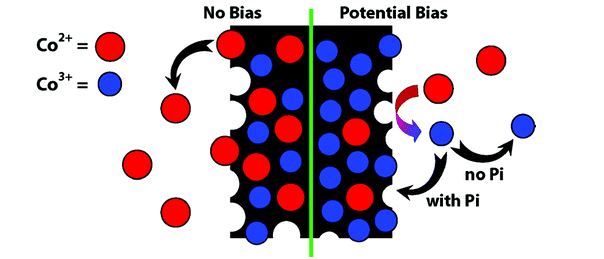
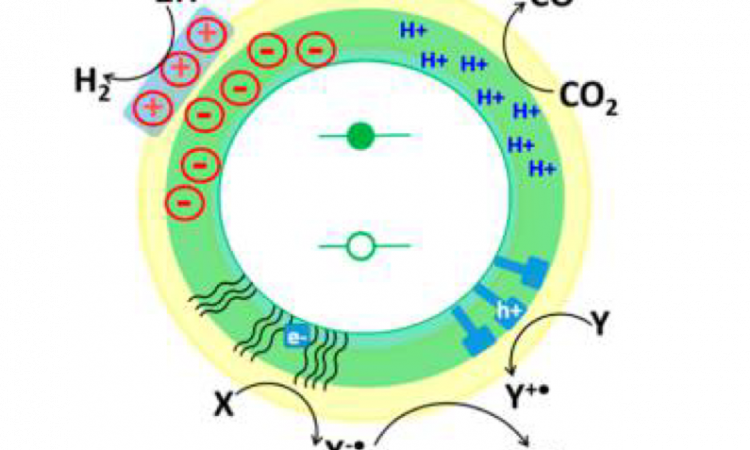
Neutral Water Splitting by Self-Repairing Catalyst
In this work the authors discussed how and why a self-healing mechanism works for their catalyst, as well as how to rationally design other self-healing water oxidation catalysts by better understanding the kinetics of the key catalytic steps.
Controlling Nano-Sized Machines to Deliver Drugs in the Body
Tiny machines fixing disease inside the body may not be science fiction for much longer! Let’s learn about making and controlling nanomotors that could one day deliver drugs from within!
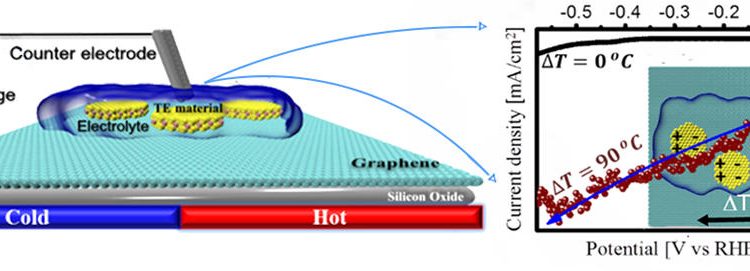
Using Heat to turn Water into Fuel
The development of clean, efficient, and renewable forms of energy is a critical scientific challenge. Plants have already figured out how to do this via photosynthesis. Can we develop a process that mimics this?
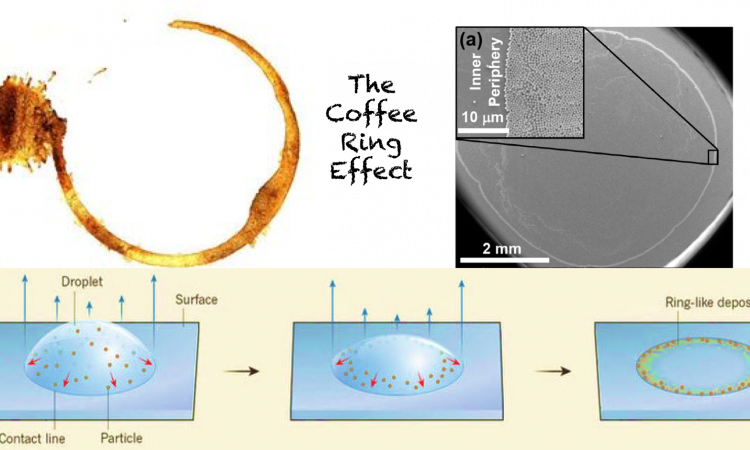
Getting Rid of the Coffee Ring
Coffee has more to offer your brain than just yawn-free days! Transform your everyday experience with coffee and its stains to an understanding of the interesting phenomenon of coffee ring effect. Explore its implications and challenges in materials industry and learn about a simple approach to get rid of it.
Versatile Surfaces: How to Enhance the Role of Quantum Dots in Photocatalysis
There are lots of ways to use sunlight to achieve sustainable energy goals. Photocatalysts, which can use sunlight to power useful chemical reactions, are of great interest for the production of solar fuels like hydrogen. Read more about how we can use novel nanomaterials as photocatalysts in this Chembite!
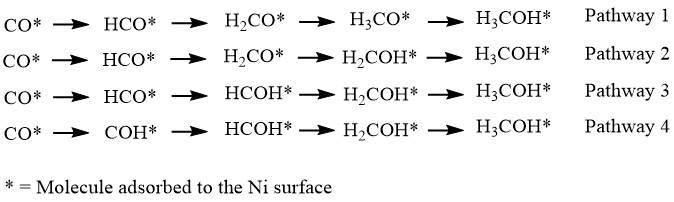
Scratching the Surface: Subsurface Hydrogen Aids CO Hydrogenation to Methanol With Nickel
In today’s Chembite we appreciate and explore some remarkable mechanistic aspects of the hydrogenation of CO at a nickel surface. The paper covered gives the first account of catalytic methanol and formaldehyde production from CO by Ni. But to explain why we need to go deeper than the surface…
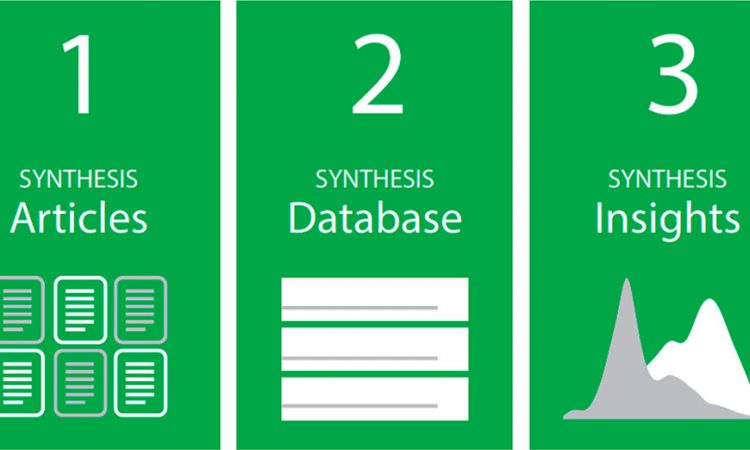
Machine Learning for Understanding Materials Synthesis
Title: Materials Synthesis Insights from Scientific Literature via Text Extraction and Machine Learning Authors: Edward Kim, Kevin Huang, Adam Saunders, Andrew McCallum, Gerbrand Ceder, and Elsa Olivetti Year: 2017 Journal: Chemistry of Materials The sheer volume of publications makes scientific literature a vast sea of information…
Bicarbonate Controlling the Rate of CO2 Reduction
This work shows that bicarbonate (HCO3-) is neither a general acid nor a reaction partner in the rate-limiting step of electrochemical CO2 reduction catalysis mediated by planar polycrystalline Au surfaces. Kinetic modeling studies and electrochemical experiments suggest that it acts as a proton donor in steps past the rate-limiting one and a buffer in the solution.
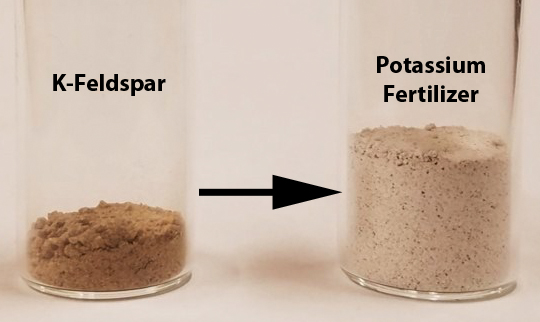
Using Green Chemistry to Make Fertilizer from Minerals
One of the most important types of chemicals in agriculture is fertilizer. This paper shows a new way to cleanly make fertilizer from a very common mineral!
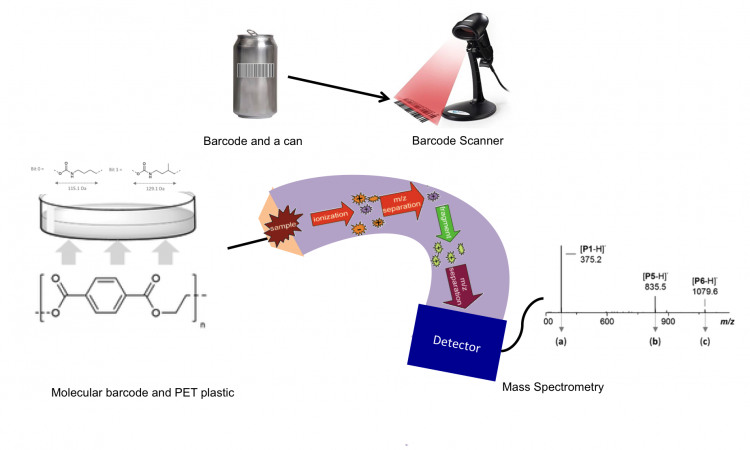
Scan your barcode, the molecular one!
What comes to your mind when you think of barcodes and plastics? This post will enlighten you on a new kind of barcode, molecular barcode, encrypted within the plastic itself!
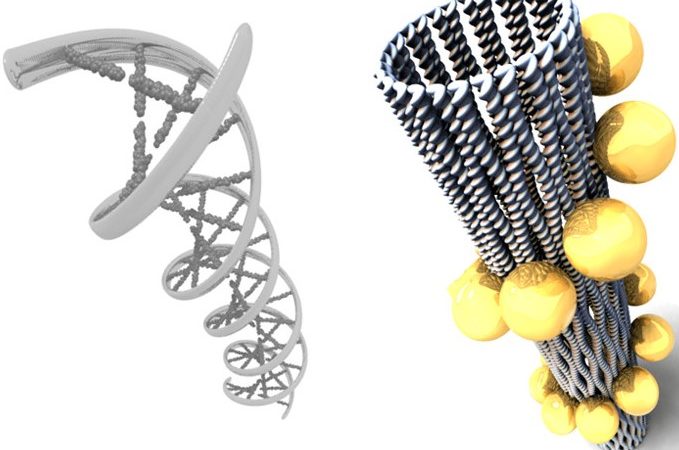
Assembling Gold Nanoparticles into 3D Structures Using DNA
How can DNA be used to enhance applications in nanotechnology? The authors here create never-before-seen optical systems by combining DNA origami with plasmonic nanoparticles.
Unveiling the Mystery of Nanomaterials
Ever wondered how and why the world of ‘nanomaterials’ is fueling the ‘big’ technological advances! Let us travel past the layers of sophisticated ingenious performance of nanomaterials and learn the fundamental reason behind their properties.